
by Dan Eden for Viewzone (http://www.viewzone.com/teflon.html)
When I was a kid, we had a blue and green parakeet named "Wardie." He could squack his name and was fond of sitting on our shoulder in the kitchen near his open cage when we ate. One afternoon I skipped school and doubled back to the house. Both of my parents were at work and wouldn't be home until dinner time. It was just me and Wardie.  I decided to cook a hotdog in the new "miracle" non-stick pan that Mom had just bought. I set the electric burner to "HI" to warm up the pan and dashed into the livingroom where I had a movie on the TV. I decided to cook a hotdog in the new "miracle" non-stick pan that Mom had just bought. I set the electric burner to "HI" to warm up the pan and dashed into the livingroom where I had a movie on the TV.I can't remember what it was, but it must have been interesting. After a couple of minutes I smelled something metallic. I ran to the stove and turned the burner off. There was no smoke. The pan had a dark brown stain that wouldn't wash away. I was in trouble now. I took it out back and hid it in the woods. I was feeling the guilt of missing school and ruining Mom's new pan when I noticed that Wardie was lying motionless on the bottom of his cage. He was dead. When my folks returned from work they were so upset about Wardie that they didn't even notice the pan was missing. I somehow knew that I had killed him, but until now I never knew how. Who knew? They knew. It turns out that someone already knew how Wardie died, along with thousands of other small pets. They knew, but they kept it quiet. To be fair, for about 50 years scientists from DuPont have published studies documenting the temperatures at which non-stick cookware coatings begin to break apart, releasing toxins into the air. They observed multiple cases ofteflon flu in their workers. Two of eight women working on the early Teflon production line gave birth to seriously deformed babies. DuPont and private labs conducted a series of studies beginning in the 1950s to identify the toxic components from heated Teflon. They killed many birds and rats in efforts to understand the potency of the gases and particles. Yet they posted no warnings on the pots, pans and kettles that could be expected to impact the public. Teflon Flu? DuPont is on record admitting that humans can also become "sick" when the pan is heated to high temperatures. According to a DuPont spokesperson, "You get some fumes, yes, ... and you get a flu-like symptom, which is reversible." Symptoms include headaches, chills, backache, and a temperature between 100 and 104 degrees. While not on the pans themselves, the "flu" warning is on the DuPont Web site.
The toxicity doesn't come from Teflon directly, but mainly from one of the many compounds that are released when the non-stick coating is heated to a high temperature. Perfluorooctanoic acid (PFOA), known as C8 (because it has 8 carbon atoms), is a proven carcinogen. Lab studies suggest that PFOA can cause cancer and birth defects in animals, and might pose a risk in humans. PFOA stays in the body for years and in the environment indefinitely. 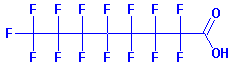 PFOA molecule. 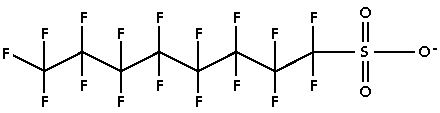 PFOS molecule. Experiments by the Envoronmental Working Group show that heating the Teflon pan to just 500°F was probably enough to kill Wardie. That temperature is hot enough to release PFOA and other toxic gases, all colorless and odorless. Browning your bacon or hotdog can easily bring the pan temperature to well over 600°F -- and perhaps even higher in "hot spots" that occur where the pot touches the heating element or flame. Because of Teflon's wide spread use over the last 50 years, you might guess that, by now, PFOA and FPOS should be inside just about every one of us. You would be correct. PFOA & PFOS in 100% of newborn's blood!  In 2007, the shocking results of a study conducted at the John Hopkin's Medical Center (Bethesda, MD) showed that levels of the toxic PFOA were found in 100% of a sample of almost 300 newborns, delivered at the hospital. Samples of umbilical chord blood were also shown to contain varying levels of PFOS. Asians (6 ng/ml) had the highest concentration, followed by Blacks (5.1 ng/ml) and Whites (4.2 ng/ml). Males babies had higher PFOS and PFOA than females. Obese and underweight mothers had slightly higher concentrations than women of normal weight.[1] Other studies have found that some of the highest levels of PFOA and PFOS have been in children.[2] In 2007, the shocking results of a study conducted at the John Hopkin's Medical Center (Bethesda, MD) showed that levels of the toxic PFOA were found in 100% of a sample of almost 300 newborns, delivered at the hospital. Samples of umbilical chord blood were also shown to contain varying levels of PFOS. Asians (6 ng/ml) had the highest concentration, followed by Blacks (5.1 ng/ml) and Whites (4.2 ng/ml). Males babies had higher PFOS and PFOA than females. Obese and underweight mothers had slightly higher concentrations than women of normal weight.[1] Other studies have found that some of the highest levels of PFOA and PFOS have been in children.[2]Two perfluorinated compounds-- PFOA and PFOS-- were found in virtually all of 299 newborn babies studied in Baltimore, according to scientists from Johns Hopkins University. The results indicate that exposure in the womb to these two chemicals is virtually ubiquitous. Eight other PFCs were detected less frequently.The saturation of PFOA in human blood has lots of scientists worried. The PFOA molecule does not degrade over time, so every one that is made and released in the environment will continue to be there for an indefinite time. The cumulative levels of PFOA will continue to increase in both the environment and in the human body.  "In 1978 PFOA was confirmed to be completely resistant to biodegradation in a study done by 3M to substantiate a similar finding in a 1972 National Academy of Sciences report [3]. The primary finding of the new 3M study -- '...the results of this study suggest that these chemicals are likely to persist in the environment for extended periods unaltered by microbial catabolism.""Perfluorinated compounds are extremely resistant to biodegradation... Although compounds with single fluorines have been shown to release fluoride ions as a result of biodegradation, perfluorinated compounds have rarely or never been shown to undergo natural degradation. For this reason, no modification of the perfluoro components of compounds in this study was anticipated." [4]So how much PFOA can humans tolerate? The answer to this question is not known. Usually a substance will be toxic in proportion to body weight or species -- the heavier the animal, the more toxin needed to cause illness or death. But this is not the case with PFOA. Toxic levels vary with gender, species and length of exposure. In truth, scientists do not understand the exact method or systems involved with this poison. But they warn against thinking of toxins as something that "turns on" at a certain level. Very low exposure of PFOA, over an extended period of time, may be altering human biology increasingly with each generation. The persistance of the PFOA molecule is the problem without a solution. How hot is too hot?  Repeated use of Teflon pans and cooking utensils causes the coating to deteriorate. Official documents show ultrafine particles start coming off the pan at 554 degrees Fahrenheit. If they get in to your lungs they can embed in the lining. At 680°F the toxic gases quickly enter the air. Repeated use of Teflon pans and cooking utensils causes the coating to deteriorate. Official documents show ultrafine particles start coming off the pan at 554 degrees Fahrenheit. If they get in to your lungs they can embed in the lining. At 680°F the toxic gases quickly enter the air.The toxic gas is a montage of molecular byproducts of perfluorinated compounds (PFC's). Studies show that thermal degradation of Teflon leads to the slow breakdown of the fluorinated polymer and the generation of a litany of toxic fumes including TFE (tetrafluoroethylene), HFP (hexafluoropropene), OFCB (octafluorocyclobutane), PFIB (perfluoroisobutane), carbonyl fluoride, CF4 (carbon tetrafluoride), TFA (trifluoroacetic acid), trifluoroacetic acid fluoride, perfluorobutane, SiF4 (silicon tetrafluoride), HF (hydrofluoric acid), and particulate matter. At least four of these gases are extremely toxic -- PFIB, which is a chemical warfare agent 10 times more toxic than phosgene (COCl2, a chemical warfare agent used during World Wars I and II), carbonyl fluoride (COF2 which is the fluorine analog of phosgene), MFA (monofluoroacetic acid) which can kill people at low doses, and HF, a highly corrosive gas. Most of these gases are colorless and have no scent. They are in the air long before your pan starts to smoke. Time To Chuck the Old Pots and Pans? Don't forget the sofa, carpet, baby's clothes...  Teflon is also known as Stainmaster, Gore-tex, Silverstone... 3M has a similar compound that degrades to PFOA in their Scotchguard fabric treatment. It's in your carpet, sofa, clothes and it coats a wide variety of cooking utensils, appliances and tools. It's all around us and in us. Teflon is also known as Stainmaster, Gore-tex, Silverstone... 3M has a similar compound that degrades to PFOA in their Scotchguard fabric treatment. It's in your carpet, sofa, clothes and it coats a wide variety of cooking utensils, appliances and tools. It's all around us and in us.Even if PFOA were banned today, the global mass of PFOA would continue to rise, and concentrations of PFOA in human blood would likely continue to build. Long after PFOA is banned, other PFC chemicals from 50 years of consumer products will continue to break down into their terminal PFOA end product, in the environment and in the human body. In fact, this warning about PFCs may be too late. One of the biggest mistakes the chemical industry has ever made! Referring to teflon applied to clothing as a stain guard, Jane Houlihan, vice president for research at the Environmental Working Group, an activist organization said, "In retrospect, this may seem like one of the biggest, if not the biggest, mistakes the chemical industry has ever made... Those chemicals can absorb directly through the skin." On the opposite side of the argument, Uma Chowdry, DuPont's VP of research and development said, "We are confident when we say that the facts, the scientific facts, demonstrate that the material is perfectly safe to use." She claims that the substance is completely safe, despite the fact that the key chemical, C-8, is in everyone's blood. "We do not believe there are any adverse health effects," she said. "There are lots of chemicals that are present in our blood." While Dupont has not embraced the idea that Teflon chemicals cause cancer or other human illness or defects, it did recently settle allegations by the EPA on Teflon with a $17 million fine, the largest civil penalty enforced and reached under any federal environmental law. In this case, Teflon chemicals were exposed to many workers and was even found passed by one pregnant woman to her fetus. This suggests that the harm to human health we encounter today will only be intensified in the coming years, as more PFOA is produced and higher levels accumulate in our blood.
"A manufactured chemical used in making nonstick and stain-resistant products may disrupt important reproductive tissues in pregnant and unborn female mice, according to researchers in North Carolina. A report in the latest edition of the journal Toxicological Sciences was the latest to find possible links between C8, or perfluorooctanoic acid (PFOA), and problems in animal health, development and reproduction." [Jeff Montgomery, Delaware News Journal.]On January 2006, the EPA advisory board of 17 scientists unanimously recommended PFOA be labelled a 'likely carcinogen' (a carcinogen is a cancer-causing substance) in humans. The EPA asked DuPont, and seven other companies that use PFOA in manufacturing processes, to phase out its use. DuPont has agreed to take steps to make sure that by the year 2015, the chemical would not be released into the environment from its manufacturing plants, and will not agree to stop using it, or to stop making Teflon. The problem for Dupont is, as it stans now, it cannot make Teflon without this chemical, though it says it is looking for a substitute
According to a report in ScienceDaily (July 6, 2010) Marianne Kraugerud's doctoral research has led to the discovery that individual variants of the environmental pollutants PCB and PFC can affect several of the body's hormone systems in a more complex way than previously supposed. Humans and animals are constantly exposed to these toxins through the food they eat and the air they breathe. Kraugerud concludes that the impact of these pollutants should be taken into account when carrying out risk appraisals of PCB and PFC.Marianne Kraugerud's thesis studies the effects of different variants of the environmental toxins PCB and PFC on sheep and on cells grown in the laboratory. Her research on sheep is of comparative interest for humans.
Polychlorinated biphenyls (PCB) and perfluorinated compounds (PFC) are chemicals that only break down naturally to a very small degree and therefore have a strong tendency to accumulate in the environment. While PCBs are known to be environmental pollutants and have not been legally produced since the 1970s, the use of many PFC variants is rapidly increasing in products such as water-resistant clothing and coatings in saucepans and frying pans. Marianne Kraugerud's thesis shows the effects of PCB 118 and PCB 153, which are two separate PCB variants with different chemical characteristics. In lambs exposed to these substances while in the womb and via their mother's milk, effects were demonstrated both on the formation of egg cells in the ovaries and on the hormones that control the function of the ovaries in female lambs. Kraugerud also found that sheep foetuses that had been exposed to these PCB variants while in the womb had a diminished ability to produce the vital hormone cortisol. Through laboratory cell cultures, Kraugerud demonstrated that both PCB and PFC can directly affect the production of steroid hormones. Steroid hormones, including for example oestrogen, testosterone and cortisol, are necessary for maintaining the capacity to reproduce, normal development and normal bodily functions in humans and animals. Since PCBs and PFCs are widespread in the environment and can affect the body's hormone systems in a more complex way than previously supposed, Marianne Kraugerud recommends that these effects are emphasised when risk appraisals on these substances are drawn up. Marianne Kraugerud, veterinary surgeon, presented her doctoral thesis on 21st June 2010 at The Norwegian School of Veterinary Science. The thesis is entitled: "Endocrine disruption by persistent organic pollutants: effect studies using in vivo and in vitro models." |
Notes:[1] Determinants of fetal exposure to polyfluoroalkyl compounds in Baltimore, Maryland. - Environ Sci Technol. 2007 Jun 1; 41(11): 3891-7 Apelberg BJ, Goldman LR, Calafat AM, Herbstman JB, Kuklenyik Z, Heidler J, Needham LL, Halden RU, Witter FR
[2] April 5 , 2003. The Columbus Dispatch (Ohio). DUPONT CHEMICAL SHOWING UP IN BLOOD OF CHILDREN, ADULTS. EPA wants to regulate the compound found in many household items. By Michael Hawthorne.
[3] Degradation of Synthetic Organic Molecules in the Biosphere, (NAS 1972). (FC-95 and FC-143).
[4] 3M. 2000. Biodegradation study of PFOS. US Environmental Protection Agency Administrative Record Number AR226-0057.
[2] April 5 , 2003. The Columbus Dispatch (Ohio). DUPONT CHEMICAL SHOWING UP IN BLOOD OF CHILDREN, ADULTS. EPA wants to regulate the compound found in many household items. By Michael Hawthorne.
[3] Degradation of Synthetic Organic Molecules in the Biosphere, (NAS 1972). (FC-95 and FC-143).
[4] 3M. 2000. Biodegradation study of PFOS. US Environmental Protection Agency Administrative Record Number AR226-0057.
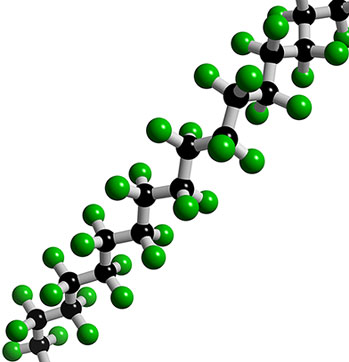 Teflon was discovered by Roy Plunkett in 1938 while he was attempting to develop a new kind of refrigerant (remember CFC's). One of the compounds polymerized while in a storage container and its non-stick properties were soon recognized as unlike any other chemical. The official patent was acquired by DuPont in 1941.Structurally, Teflon consists of a chain of carbon atoms which, if they are not sticking to eachother, are stuck to fluorine atoms. The flourine atoms are stuck so tightly to the carbon chain that virtually nothing can detach them. As a result, nothing can attach itself to the molecule -- nothing.
Teflon was discovered by Roy Plunkett in 1938 while he was attempting to develop a new kind of refrigerant (remember CFC's). One of the compounds polymerized while in a storage container and its non-stick properties were soon recognized as unlike any other chemical. The official patent was acquired by DuPont in 1941.Structurally, Teflon consists of a chain of carbon atoms which, if they are not sticking to eachother, are stuck to fluorine atoms. The flourine atoms are stuck so tightly to the carbon chain that virtually nothing can detach them. As a result, nothing can attach itself to the molecule -- nothing. Federal researchers have asked a respected scientific journal to pull from its Web site a government-sponsored study that warned Americans could be exposed to C8 and similar chemicals when they eat chicken eggs.
Federal researchers have asked a respected scientific journal to pull from its Web site a government-sponsored study that warned Americans could be exposed to C8 and similar chemicals when they eat chicken eggs.
No comments:
Post a Comment